Werner Heisenberg
quantum mechanics
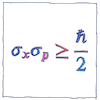
|
Uncertainty principle
Werner Heisenberg declared a limit to what can be determined precisely. We don’t have this problem when talking about planets or billiard balls, only for elementary particles such as photons or protons. The more we know about one property of a particle, such as its position, the less we know about any related property, such as its momentum.
Half ħ
The standard deviations of position and momentum multiplied together is greater than or equal to half the reduced Planck constant.
Unsure
You’d think this’d be easy but I’m not sure.
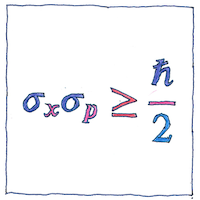


Heisenberg’s uncertainty principle is a fundamental property of quantum systems, not merely a limit to how small a thing we can measure.
The illustration represents the work of Earle Hesse Kennard and Hermann Weyl. The standard deviation of position (sigma sub-x) multiplied by the standard deviation of momentum (sigma sub-p) is greater than or equal to half the reduced Planck constant.
See also in The book of science:
Readings in wikipedia: