John Dalton
physics

|
Atomic theory
invisible & irreducible atoms remained controversial since Democritus in the fifth century until John Dalton seeing that common chemical processes produced elements in fixed proportions— so much hydrogen per oxygen in water so much oxygen per carbon in carbon dioxide claimed that chemical elements are made of atoms atoms of the same element have the same weight atoms of different elements have different weights atoms combine in only small whole-number ratios to form molecules (which he called “compound atoms”) and atoms cannot be created or destroyed (by chemical processes).
Notation
Describing atoms
as spherical bodies,
Dalton proposed a uniform notation
for atoms and molecules
—each a circle
containing a letter or decoration.
These circles formed an alphabet
which chemists could use to spell
more complex compounds.
His circle for hydrogen
contained a dot—
same as the Egyptian hieroglyph
for the sun, ‘ra,’
which had not yet been decyphered.
Symbols
If an atom cannot be destroyed, then these are not atoms— lives, families, languages, tribes, happiness, peace of mind, faith, belief, hope. If I were to symbolize my love with a plus-sign and put that sign in a circle, then I would be trying to say my love cannot be destroyed.
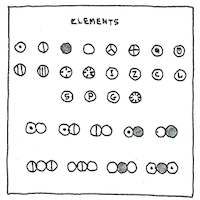


Before we had atomic force microscopes, the existence of atoms and molecules had to be deduced from chemical experiments.
Dalton was the first to assemble the evidence into a complete theory, and the first to prepare a table of the atomic weights of known elements and molecules. As a poet, I am impressed that he invented an alphabet to symbolize the elements and molecules. These are more interesting and artistic than the two-letter chemical symbols based on the Greek that we use today.
My editorial insertion is the parenthetical “(by chemical processes)” in the list of Dalton’s claims, since we know now that an atom may be destroyed.
Around 1787, Dalton rediscovered George Hadley’s theory about trade winds. In 1801 he published a book on English grammar. Dalton suffered a less common form of color-blindness, deuteranopia, and was the first to describe and explain color blindness, proposing that it was due to a discoloration of the liquid medium of the eyeball, which was wrong. In his honor color blindness is sometimes called Daltonism, and the unit of atomic mass the dalton.
See also in The book of science:
You can find lots of information about John Dalton and atomic theory: