Michael Faraday
physics
|
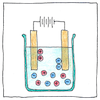
Laws of electrolysis
1. Running a direct electric current through a solution breaks molecular bonds and attracts separated elements to opposite electrodes. 2. The mass of a compound separated by electrolysis is directly proportional to the quantity of electricity applied to it. Moreover, the mass of altered elemental material is directly proportional to the element’s equivalent weight.
History of electrolysis
In 1785, Martinus van Marum used his static electricity generator, storing a charge in large Leyden jars, to reduce tin, zinc, and antimony from their salts. Starting in 1800, many scientists, including William Nicholson, Anthony Carlisle, and Johann Wilhelm Ritter, followed by Henry Cavendish and Antoine Lavoisier, found that static electricity separated water into hydrogen and oxygen. In 1807, Humphry Davy, working with Michael Faraday, used the Royal Society’s voltaic pile to discover potassium, sodium, barium, calcium, and magnesium. In 1808, Humphry Davy tried to produce aluminium from the mineral alumina, but was not successful. In 1825, Hans Christian Ørsted produced an impure form of it. Friedrich Wöhler finally isolated it in 1827. Wöhler was also the first to isolate yttrium, beryllium, and titanium. In 1812, Michael Faraday decomposed sulfate of magnesia; in 1825, he discovered benzene, and in 1834 he published his laws of electrolysis.
Periodic
Quite a few elements had yet to be discovered. Some had been suspected from lines in spectrograms. Some have been recognized as a common component of various alloys. Standard chemical analytic techniques continued to fail. Scientists continued to try different solutions. Eventually, all the gaps were filled in. But rejoice! Everything is not already known!
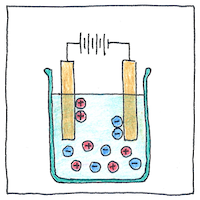


Dmitri Mendeleev predicted the existence of gallium, based on his invention of the periodic table. Paul-Émile Lecoq de Boisbaudran identified it in a sample of mineral ore using a spectroscope and isolated it using electrolysis.
See also in The book of science:
Readings in wikipedia: