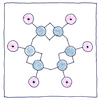
Benzene ring
 One atom of carbon usually bonds to four hydrogen atoms,
but benzene has only one hydrogen for each of its six carbons.
The remaining bonds must be consumed in its structure;
otherwise, benzene would stick to everything, or fall apart,
which is not its observable behavior.
The six carbons use their three remaining bonds
to hold on to each other, but the arrangement
was unknown, until it came in a daydream
to August Kekulé, of a snake
forming a circle by biting its own tail,
which is strange because a snake
has only its mouth for grasping. I’d rather the dream
were of a monkey grabbing with both hands and feet.
It may be difficult to distinguish the dream
from the waking interpretation. Put six carbons
in a ring, join them hand to hand,
and let each grab the hand of a hydrogen.
This still leaves one hand per carbon.
Given a circular symmetry, various cross bonds
had been proposed, but Kekulé realized
alternating carbon-to-carbon bonds were doubled.
The carbons grasp two hands on one side only.
One atom of carbon usually bonds to four hydrogen atoms,
but benzene has only one hydrogen for each of its six carbons.
The remaining bonds must be consumed in its structure;
otherwise, benzene would stick to everything, or fall apart,
which is not its observable behavior.
The six carbons use their three remaining bonds
to hold on to each other, but the arrangement
was unknown, until it came in a daydream
to August Kekulé, of a snake
forming a circle by biting its own tail,
which is strange because a snake
has only its mouth for grasping. I’d rather the dream
were of a monkey grabbing with both hands and feet.
It may be difficult to distinguish the dream
from the waking interpretation. Put six carbons
in a ring, join them hand to hand,
and let each grab the hand of a hydrogen.
This still leaves one hand per carbon.
Given a circular symmetry, various cross bonds
had been proposed, but Kekulé realized
alternating carbon-to-carbon bonds were doubled.
The carbons grasp two hands on one side only.
Discovery and proof
Discovery and proof follow independent paths
in the mind. Mathematicians leap
to conjectures that may take years to reach
by means of proof. No one can say
why the idea pops into the mind of one
and not another, except that one must ponder
the problem. There are methods,
but none are sufficient. Some say genius
is dreaming, but a dream is effortless
and unconvincing, whereas the opposite
is true of the tedious and pedantic.
Locking rings
I learned to unlock and lock the Chinese ring puzzles
my uncle bought for me in San Francisco Chinatown.
Then I started linking triangular with circular,
and large with small, until every pair was linked
with all the other pairs to the extent that every ring
fit in all the others in a single mass
of snakes writhing in a perpetual metal orgy.

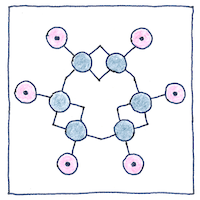


Understanding the structure of benzene helped make all aromatic compounds more understandable and useful. It is probable that Kekulé told the story about day-dreaming of the ouroboros as a joke, because his discovery came after years of studying compounds with carbon-to-carbon bonds. Today, there is controversy over whether single and double carbon bonds alternate, which would helps explain the thermodynamic stability that contributes to its aromatic properties. If they do not alternate, then the electrons could be distributed equally between the six carbon atoms, or spin-coupling could contribute to its aromatic properties.
See also in The book of science:
Readings in wikipedia: