Michael Faraday
chemistry
|
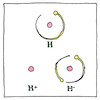
Ion
Michael Faraday knew electrolysis carried some kind of particle to one electrode or the another. His friend William Whewell suggested naming this kind of particle “ion,” Greek for the verb meaning going.
Charged particles
Normally, in an atom, each proton, which has a positive electric charge, is exactly balanced by an electron, which has a negative electric charge. If an atom has more electrons than protons, it has a net negative charge. It’s attracted to the anode of a battery, so Faraday called this an “anion.” If an atom has fewer electrons than protons, it has a net positive charge. It’s attracted to the cathode of a battery, so Faraday called this a “cation.” During electrolysis, a substance is dissolved by a solvent, which produces either anions attracted to the positive anode or cations attracted to the negative cathode.
Faraday cup
Transient and fragile particles and any extra electrons are detected by a Faraday cup as they exit the target end of a particle accelerator. These devices are finely tuned to show single elementary electric charges each about 1.602 times 10 to the minus 19 coulombs. Faraday didn’t invent the Faraday cup, but he explained that electrolysis moves ions and observed that ions of the same charge repel each other.
Going
I have the spring and clockwork of a four-wheeled toy that I wind up, set on the floor, and let go— instant joy, simple as going. But when a good friend dies, it’s hard not to refuse the pleasures of this world— too fragile, too temporary.
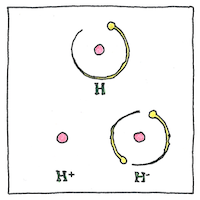


An ion may be monatomic or polyatomic. A household air ionisers give air molecules a negative charge so that, floating around a room, they attach themselves to positively charged particles of dust or air-borne bacteria so that they are more readily removed from circulation.
See also in The book of science:
Readings in wikipedia: