Jöns Jacob Berzelius
chemistry
|
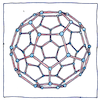
Allotropy
Chemists distinguished elements by their weights but the chemistries of some continued to be squirrelly. Knowing nothing at the time about polyatomic molecules or crystal structures, Friedrich Wöhler and his friend Berzelius had proposed that chemical compounds could have different isomers, but they believed that pure elements were inherently monoatomic. When it was discovered that different derivations of a pure element could have different chemical behaviors, Berzelius coined the term meaning other behavoir to describe the inherent variations of an element.
Allotropes
Carbon and sulfur have the most known allotropes. Gold and the noble gases have none. Only elements in groups 13 through 16 of the periodic table have natural allotropes. In group 13 only boron. In group 14 carbon and tin. (Germanium’s allotrope germanene doesn’t exist in nature). In group 15, phosphorus, and arsenic. (Explosive antimony can be made in the lab). In group 16, oxygen, sulfur, and selenium. At extremely low temperatures, diatomic hydrogen, and under extreme pressure maybe metallic hydrogen.
As for why he does the things he does
We say he’s crazy, cuckoo, cockeyed, mad, a few cards short of a full deck, demented, deranged, disturbed, daft, daffy, dippy, dotty, potty, a nutcase, a weirdo, unbalanced, unhinged, loco, loony, barmy, loopy, whacky, screwy, addlebrained, bonkers, bananas, fruity, batty, buggy, and off his trolley, but as for why he does the things he does, God only knows.
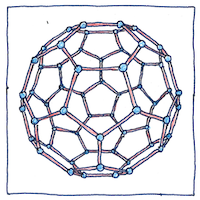


It should be emphasized that Berzelius didn’t know why an element would have different chemical behaviors. He apparently assumed the different behaviors were inherent characteristics of the atoms themselves.
See also in The book of science:
Readings in wikipedia:
Other readings: