Josiah Willard Gibbs
thermodynamics

|
Chemical potential
In a thermodynamic system, potential energy can be absorbed or released during a chemical reaction or phase transition, or, if everything’s in equilibrium, then the potential energy is zero.
Terms
Josiah Willard Gibbs examined the energy of a system in terms of its entropy, volume, pressure, and temperature, and formulated how adding a molecule affects its energy. He coined the term enthalpy. * Gibbs coined the term statistical mechanics. He created a more general formulation of the statistical properties of systems of many particles than James Clerk Maxwell or Ludwig Boltzmann.
Pills
The three weird sisters boil poisoned entrails for a charm. We toss back aspirin and probiotics. Fish is brain food; fructose is energy food. Each pill, we feel, helps quantify our future.
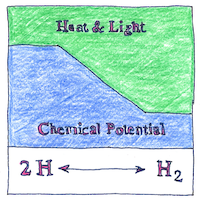


Chemical potential enabled elegant formulations for the behaviors of thermodynamic systems, such as the phenomenological fundamental equation of thermodynamics, which I won’t try to reproduce or explain here.
See also in The book of science:
Readings in wikipedia:
Other readings: