Emil Adolf Hermann Fischer
chemistry

|
Enzyme specificity
Hermann Emil Adolf Fischer, working on the structures of the isomers of sugar, found that enzymes that can convert one sugar into alcohol cannot convert another and realized that all enzymes are keyed to specific molecules and that living cells use enzymes as catalysts for specific purposes.
Sugars
Simple sugars— glucose, whose right-handed form is essential to cells and is our primary metabolic fuel, fructose or fruit sugar, xylose, the main building block of plant walls, arabinose and ribose, from gum arabic deoxyribose, lyxose, xylulose, ribulose, allose, altrose, mannose, gulose, iodose, talose— and the compound sugars— sucrose, common table sugar, the compound of fructose and glucose, maltose or malt sugar, the compound of two glucoses, lactose or milk sugar, the compound of galactose and glucose, lactulose, trehalose, cellobiose kojibiose, nigerose, isomaltose, sophorose, laminaribiose, gentiobiose, turanose, maltulose, palatinose, gentiobiulose, mannobiose, melibiose, melibiulose, rutinose, rutinulose, xylobiose— are not equally sweet—fructose is the sweetest sugar— inviting comparisions to other things in life.
Fischer projection
Fischer taught how to draw the acyclical form of a simple sugar so that the handedness of each chiral carbon is clearly represented and information about its three-dimensional form is not lost as if you were looking at the molecule (actually invisible, being less than a nanometer) and could distinguish the branches, right from left, up from down, and front from back. We might in fact have figured each other out. It’s hard to tell. Thousands of experiments over years will be needed to distinguish the nuances.
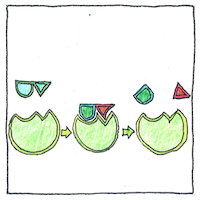


Enzymes increase the rate of biochemical reactions. I became fascinated with the structures of sugars and lost sight of the fact that they are useful because enzymes can break them up.
Sucrose (C12H22O11), a disaccharide, is a combination of two monosaccharides, glucose and fructose (both with the chemical composition C6H12O6). What distinguishes glucose from fructose is their chiral forms. Chiral carbon structures are asymmetrical, like the thumb on a hand; in sugars, a hydrogen sticks to one side and an oxygen-hydrogen pair sticks to the other.
The disaccharide of glucose is maltose (C12H22O11). Brewer’s yeast produces the enzyme Saccharomyces cerevisiae to convert maltose during the brewing of beer into ethanol (C2H5OH) and carbon dioxyde (CO2) .
See also in The book of science:
Readings in wikipedia: