Victor Goldschmidt
geophysics

|
Goldschmidt classification
Atmophile “vapor-loving” elements are volatile and fill Earth’s atmosphere. Lithophile “rock-loving” elements combine with oxygen and form the rocks of Earth’s crust. Chalcophile “chalcogen-loving” elements but what is a chalcogen? Chalcophile elements combine with sulfur and remain in Earth’s crust. Siderophile “iron-loving” elements dissolve in iron and sink into Earth’s molten core.
Meteoric
The abundance of elements in planet Earth is assumed to be similar to chondrite meteorites, formed out of same cloud of dust as the rest of the solar system. But geological processes on Earth have boiled away volatile elements and buried heavier elements resulting in appreciation for geochemical sleuths like Victor Goldschmidt.
After the performance
Things get sorted out after the curtain falls. Supporting actors go out for a drink; the lead actors retreat to rest for the next curtain. People in the audience, affected, or enabled, or relieved, or enlightened, can try to live their lives as though things matter even more.
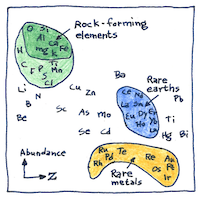


In describing how the elements become distributed on planets, Victor Goldschmidt became the father of modern geochemistry.
Earth’s atmosphere is mainly diatomic nitrogen. Earth’s crust is mainly silicate rocks. When silica crystalizes with oxygen, it forms quartz. Earth’s mantle is mainly silicate rocks rich in iron and magnesium. Earth’s core is mainly iron.
See also in The book of science:
Readings in wikipedia: