Richard Towneley, Henry Power, Robert Boyle
chemistry

|
Boyle’s law
For a given mass of confined gas, if you keep its temperature the same, its pressure times its volume is constant. So had Richard Towneley and Henry Power asserted, and so, with the help of Robert Hooke, Robert Boyle established by experiment and published in 1662.
Atomic kinetic thermodynamics
Towneley and Power used a barometer and made their observation of air at different altitudes up Pendle Hill. Hooke built an air pump for Boyle with which they experimented with different pressures and volumes. Isaac Newton showed mathematically, for an elastic fluid at rest, that density is proportional to pressure. Daniel Bernoulli derived Boyle’s law from Newton’s laws of motion applied to the movements of molecules. John James Waterston generalized the equation by showing that pressure times volume divided by temperature is constant. Boyle’s law was the first step in the kinetic theory of gases, but at the time they thought that air was one of the four elements. Much later, physicists still thought that the existence of atoms and molecules was hypothetical and controversial.
Ideal gases
You can sometimes see a thing even though you don’t believe it, and, seeing it, understand its essence, not as it actually is, transparent and invisible, but like a glass clock, whose glass gears turn incrementally to count the seconds. However, such events are rare and magical. They can’t be counted on and won’t be believed. Only years later can we distinguish the genius from the madman.
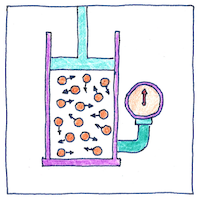


John Dalton proposed his atomic theory in 1808. Robert Brown discovered Brownian motion in 1837. The controversy over the existence of atoms was not settled until 1908 by Ludwig Boltzmann, Albert Einstein, and Jean Baptiste Perrin.
The works of Bernoulli (1738) and Waterston (1843) were ignored until James Clerk Maxwell, James Prescott Joule, Rudolf Clausius, and Ludwig Boltzmann established the kinetic theory of gases by 1871.
See also in The book of science:
Readings in wikipedia: