Michael Faraday
physics
|
Liquefaction of gases
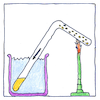
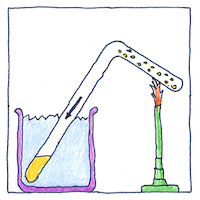
Michael Faraday showed the relation between liquids and their gases, liquifying chlorine, euchlorine, nitrate of ammonia, hydrochloric acid, sulfurous acid, hydrogen sulfide, and carbonic acid by drawing gases into bent glass tubes, or by sealing liquids in bent glass tubes including in them iron and platinum catalysts or a little water or mercury at one end, and vaporizing them by heating, and cooling by evaporation or salted ice. He made these experiments with considerable risk as tubes of gases under pressure spontaneously exploded.
Processes
You can liquify ammonia gas by compressing it, which releases heat, or you can heat liquid ammonia to convert it to a gas. If you let liquid ammonia evaporate under low pressure, it sucks heat from its environment, and if you cool that gaseous ammonia it condenses back to a liquid. If you release a condensed gas to the environment, in expanding, it sucks heat from its environment, and if you compress the gas, it releases its heat. Therefore, we see a relationship between the state of a chemical and the energy latent in it.
Liquefaction of water
It’s raining in Seattle. These clouds were raised from the Pacific and are blowing over us toward a low-pressure zone near Omaha. Great cummulus clouds move grandly to reveal random patches of blue sky. When I say “it’s raining,” I mean that we see scattered wet spots. Innumerable drops speckling windows, windshields, yards, roofs, trees waving in a gentle breeze, sidewalks, baseball caps, and bluejeans evaporate and blow away.


Processes that we observe around us every day do not teach us everything that we need to know about the nature of elements; however, the behaviors of the chemical H2O cycle through the gas, liquid, and solid phases visible in clouds, rain, rivers, lakes, bays, snow-capped mountains, and the ice cubes in our chilled drinks.
See also in The book of science:
Readings in wikipedia:
Other readings: