Gilbert N. Lewis
physics
|
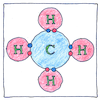
Chemical bond
An electron buzzing between two nuclei attracts them toward each other. * Atoms stick together, forming molecules, when they share electrons. * But nuclei are kept apart because the electrons need their space.
Theories
Isaac Newton realized particles adhered not by reason, nor by hooks, nor by conspiring motions. But a force, strong at small distances and weak farther apart, is responsible for chemical operations. Jöns Jakob Berzelius thought that electropositive atoms and electronegative atoms combine to bind atoms together. Edward Frankland and others thought that compounds were joined by the attraction of their positive and negative poles.
Likes
“Attracts them toward each other,” like couples? “When they share electrons,” like families? “Need their space,” like people?



Berzelius and Lewis were both right. Both electrostatic forces between atoms with opposite charges, and electrons shared between atoms bind atoms into chemical compounds.
See also in The book of science:
Readings in wikipedia: